Launch with exhaustive Target Operating Model defined
Launch under constraints, not initially designed to be commercially efficient
The Target Operating Model is structuring the execution of the launch
- Tax Optimization (Change product flows, Distribution model, affiliate structure, transfer pricing)
- Packaging issue (sustainability, temperature sensitivity, cost, convenience for patients)
- Procurement issue (excipient or material used in R&D phase is difficult to source in large scale or at reasonable cost)
- Batch size issue (to be reduced or will create too many expired products based on current shelf life)
| Topic | Time to change | Patient perception | Impacts | Company profits |
|---|---|---|---|---|
| Tax Optimization | 2/3 years | From 35% to 14.5% taxes, VAT cash flow | 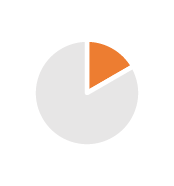 |
|
| CMO | 2/3 years (Tech Transfer) | Batch size flexibility generating or not excess & obsolescence’s, so less gross margin | 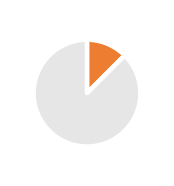 |
|
| Packaging | 0.5/2 years | 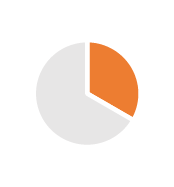 |
Convenience, size, material, Carbon footprint, Combined products (drug & medical device like syringes) | 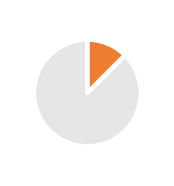 |
| Sourcing | 0.5/2 years | 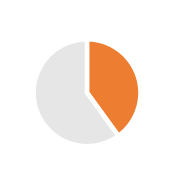 |
Unsecured sourcing, long lead-times, subject to transport uncertainty, risk of supply disruption | 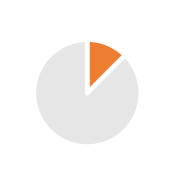 |
| Distribution | 1/2 years | 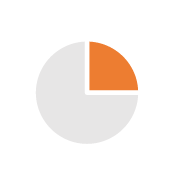 |
Efficient distribution model, Geographic expansion & stability anticipated, end to end supply chain visibility, Customer Service Level | 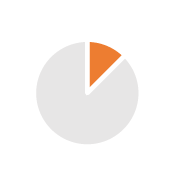 |
| Company processes | 1/2 years | Moving from complexity to simplicity, Portfolio optimization, more efficient process, shift hand work to brain work |  |
The Pivotal Role of Coordinating Functions in Designing a Robust Pharma Supply Chain
In the intricate landscape of pharmaceuticals, the design of a supply chain demands a harmonious symphony of coordinated efforts across various functions. This collaboration not only ensures a seamless and efficient flow of products but also guarantees compliance, quality, and customer satisfaction. At the helm of this harmonization stands the Project Management Office (PMO), orchestrating cross-functional collaboration to create an end-to-end Supply Chain that supports long-term profitability.
Importance of PMO: Coordination and Cross-Functionality in Design
The Project Management Office (PMO) serves as the nucleus of coordination, bridging the gaps between diverse functions involved in crafting a Life Sciences Supply Chain. A well-structured PMO aligns objectives, drives cross-functional collaboration and streamlines communication, ultimately leading to an integrated Supply Chain design that is not only operationally effective but also adaptable to dynamic market conditions.
Key Interactions and Impacts on the Target Operating Model (TOM)
- Regulatory Affairs (RA): The significance of RA cannot be overstated. It operates on two strategic levels: the Marketing Authorization Application (MAA) stage, where strategic regulatory decisions are made, and the operational level, concerning distribution licenses. Coordinating with RA ensures compliance with regulations, timely approvals, and a clear understanding of regulatory requirements that shape product(s) flows.
- Finance, Tax, and Legal: Financial considerations, tax implications, and legal frameworks interweave deeply with Supply Chain design. The coordination of these functions guarantees that the Supply Chain is aligned with fiscal strategies, international tax regulations, and legal constraints, mitigating financial risks and legal pitfalls.
- Quality Assurance (QA): QA forms the bedrock of product quality. Collaborating with QA ensures that the processes are built with rigorous quality control measures at every stage. This helps prevent deviations, reduces the risk of recalls, and safeguards patient safety.
- Sales/Marketing: The Supply Chain design must be synchronized with sales and marketing strategies to avoid overstocking or stockouts as well as defining Roles and Responsibilities between the teams. Effective collaboration here ensures that Supply Chain decisions are aligned with product demand projections and service level expectations.
- Supply Chain: At the core of the design, the Supply Chain function identified the most optimal physical movement of products. Close interaction with all other functions leads to the implementation of the right Supply Chain adapted to your product and constraints.
In this interconnected web of functions, the success of pharmaceutical Supply Chain design requires cohesive teamwork. Each function brings its unique perspective and constraints leading to the identification of the right model to operate.
The journey of designing a pharmaceutical Supply Chain requires more than isolated efforts. It demands the collective intelligence, collaboration, and coordination of various functions, all directed by the guiding hand of the PMO. This approach not only results in a resilient and adaptable Supply Chain but also reinforces the industry’s commitment to delivering safe, effective, and high-quality pharmaceutical products to patients worldwide.
Start early to set key success factors
From a few hours to full time workload
From design to implementation
ScopingDuration: 3 to 4 months
|
Execution, implementation & go-liveDuration: 9 to 12 months
|